Credit: Angewandte Chemie International Edition (2024). Doi: 10.1002/Anie.202421107
Lithium air cells can exceed the conventional lithium -ion battery by storing a lot of energy with the same weight. However, those high -performance values have remained theoretical so far, and their life is too short.
New studies released in Angewandte Chemie International Edition suggested that Chinese teams would add solute catalysts to electrolytes. It promotes charge transport and functions as a redox mediator against electrode passion.
In contrast to the lithium-ion “pushed in” between two electrodes, and the lithium battery (Li-O2) is in contrast to the lithium-ion battery, which uses anode made of metal lithium. When the battery is used, the lithium ions are dissolved and the air flows to the porous cathode aggressively charged.
Oxygen is oxidized and binds to lithium peroxide (LI2O2). When charged, oxygen is released, lithium ion is returned to metal lithium and deposited in anode. Unfortunately, the theoretical performance of such a battery is not a reality.
In fact, the effects known as overpotical delay the electrochemical reaction. The formation and decomposition of insoluble LI2O2 is slow, and its conductivity is very low. In addition, the pores in the cathode tend to be clogged, and the high potential required for the formation of oxygen decomposes electrolytes and promotes an incredible side reaction. This allows the battery to lose most of the performance after the slight charging/discharge cycle.
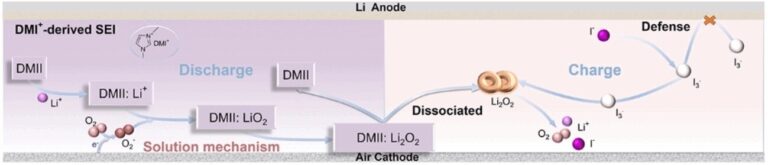
In cooperation with Dalian Maritime UNIVERSITY, the team led by the Dalian Chemical Physics of CAS is currently led by Zhong-SHUAI. Addition of Umuuzon, DMII) Is proposed. It functions as a catalytic and redox mediator to enhance performance and life.
Salt ions (i-) can easily react to form i3- and return again (redox pair). In this process, the electrons are transferred to oxygen (discharge) and they are regained (charge). This promoted charge transport will accelerate the reaction, reduce the extremity of the cathode, and improve the emission capacity of electrochemical cells.
DMI+ions from salt contain a ring made from three carbon and two nitrogen atoms. This ring has electrons that move freely, “captures” lithium -ions during emissions and effectively transmitted them to oxygen in the cathode.
In addition, DMI+ions form a very stable but very stable surf film to prevent direct contact between electrolytes and lithium surface, minimize electrolytes, and prevent side reactions. This stabilizes anode and increases the life of the battery.
The electrochemical test cells generated by the team are very promising, very low -potential (0.52 V), 960 hours of stability, and the very reversible Li2O2 formation/decomposition of LI2O2 without side reaction. It is shown.
Details: Jing Liu et al, dual-functional dual-functional Imidazorill Mediators are directed to the Li-O2 battery of long-term Li-O2 batteries, low-term Li-O2 battery, and an angeWandte Chemie International Edition (2024). Doi: 10.1002/Anie.202421107
Quoted: Lithium air battery: Acquired from January 28, 2025 https://techxplore.com/news/2025-01-Lithium-AIR -AIR-AIR-AIR-AIR-AIR-AIR-AIR-AIR-AIR-AIR-AIR-AIR-AIR-AIR-AIR-AIR-AIR-AIR-AIR-AIR-AIR-Air. -Batteries-Catalyst-LifeSpan.html
This document is subject to copyright. There is no part that is reproduced without writing permission, apart from fair transactions for private research and research purpose. Content is provided only by information.