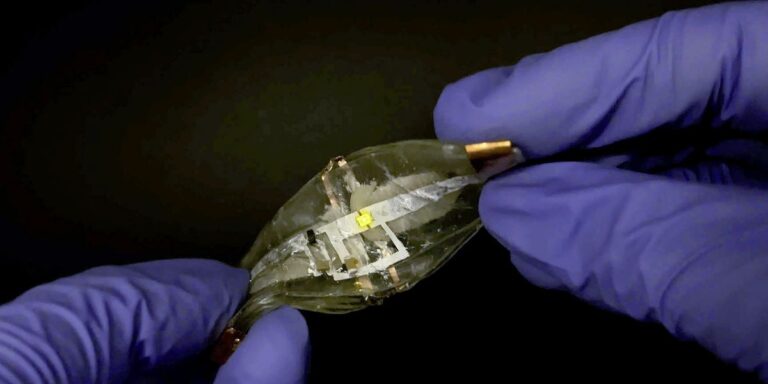
The new lithium-ion battery can not only withstand stretching and twisting, but can also be stinged with a needle and cut in half with a razor blade. And it heals itself and continues to power the device.
Wearable electronics, soft robots, and other devices can benefit from soft, stretchable lithium-ion batteries. However, most commercially available lithium-ion batteries are sealed in a hard package to eliminate moisture that can reduce performance and to prevent leakage of toxic and flammable electrolytes.
Previous studies have investigated stretchable batteries using hydrogels as electrolytes. By using water as a solvent, these hydrogel batteries prove to be less moisture and less sensitive to moisture than commercial lithium-ion batteries. However, previous devices suffered from many limitations. Some have proven stable only at relatively low voltages. Others relied on toxic and expensive florin-loaded compounds.
In a new study, the researchers developed a new water-based hydrogel lithium-ion battery that does not contain fluorine. “Many of the materials we used are non-standard materials from traditional lithium-ion batteries,” says Peisheng, a postdoctoral researcher in mechanical engineering at the University of California, Berkeley. “We have overcome these challenges by learning failed tests, building manufacturing and testing protocols and constantly improving lessons.”
The previous stretchy batteries are sturdy, surviving, creating a twist, and even proven strikes from the hammer. The new battery not only withstands stretch, twist and folding, but also shows another level of durability, even when repeatedly drilling holes with the needle, not only keeps moving yellow light on a continuous basis. Additionally, “You can cut the battery in half, collectively and self-heal to maintain more than 90% of its capacity,” said Liwei Lin, professor of mechanical engineering at the University of California, Berkeley.
New durable hydrogel lithium-ion battery
The new battery has proven stability up to 3.11 volts. Previous hydrogel batteries only had stability of up to 1.23 volts. Additionally, over a month, it was able to operate steadyly for over 500 cycles of charge and discharge, ejecting 95% of the charge received on average.
“By integrating the battery into the wristband of your smartwatch, you can multiply the battery capacity of your watch, which means you need to charge it at least once a week,” says Anju Toor, assistant professor of materials science and engineering at Georgia Tech.
Scientists warn that the energy density of batteries is only about a tenth of the lithium-ion batteries on the market. “There’s something to do,” he says. “Option of electrode structures, large-capacity electrodes and electrolyte chemistry is expected to further improve future energy density.”
Still, “even without these improvements, the current prototype is enough to replace the polymer bands of the smartwatch to provide additional power,” says Lynn.
Researchers are now trying to push the technology from lab-scale manufacturing to industry-level high-throughput manufacturing through roll-to-roll processes, says Toor.
Scientists detailed their findings on April 9 in the Journal of Science Advances.
From the article on the site
Related articles on the web